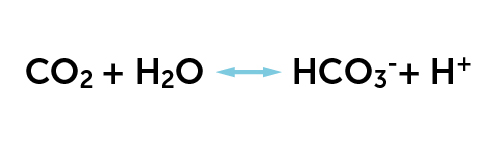
Nearly 70% of CO2 is transported in the blood in the form of bicarbonate (HCO3–). Bicarbonate is formed when CO2 chemically reacts with water (H2O) to form carbonic acid (H2CO3). Carbonic acid dissociates into bicarbonate (HCO3) and H+ (protons) by the chemical equation described in Figure 13 (formula).
The arrows pointing in both directions indicate that this reaction goes in both directions, that is, it is reversible. The reaction taking place in the body tissues where CO2 is produced is shown in the equation from left to right. When the blood arrives at the alveoli, the equation goes the other way and CO2 diffuses out of the blood. When that happens, bicarbonate (HCO3–) and H+ are combined to produce CO2 and water (H2O). CO2 is expelled from the lungs during expiration.
This chemical reaction occurs both in blood plasma and inside the red blood cells. Inside the red blood cells, this reaction occurs thousand times faster than in the blood plasma because the red blood cells contain the enzyme carbonic acid anhydrase which catalyzes the production of carbonic acid (H2CO3) from CO2 and H2O and vice versa.
The definition of an acid is a substance that produces H+. As we can see, carbonic acid dissociates into bicarbonate and H+ in Fig. 13. It is important to note that H+ is part of this reaction, and the figure thus tells us that there is a relationship between CO2 and acid in the blood. If the CO2 level in the blood increases, the amount of carbonic acid also increases, and so does the H+. It is the concentration of free H+ (ie those not combined with a negatively charged anion) which determines the pH. Thus, the more CO2, the more acid, the more H+, the lower the pH. A logical consequence of this is that respiration helps regulate blood pH. By blowing out CO2 the H+ is combined, (Fig. 13). Thus, H+/acid disappears from the blood and the pH increases. This is an example of acid-base regulation. Acid-base regulation is more thoroughly reviewed in the E-compendium of renal physiology.
Figure 13 needs to be revised.