The air pressure at sea level is about 1 atmosphere (atm) or 1000millibars, and varies depending on altitude, temperature and other environmental conditions. This pressure corresponds to about 760 mmHg. But what is pressure?
Imagine a tight container,into this you put pure oxygen gas. All molecules are in motion (except at a temperature of absolute zero, at -273°C). Oxygen molecules are bouncing around inside the container, colliding with each other, and the container walls. It is these collisions against the wall of the container that creates pressure; If you reduce the container volume (for example, by pushing down using a piston),the gas molecules are in a smaller space so will now collide more frequently with the wall,therefore the pressure inside increases. Reducing the volume for a given amount of gas molecules, creates a higher pressure. Conversely, a larger volume, which allows greater distance between gas molecules, results in a lower pressure
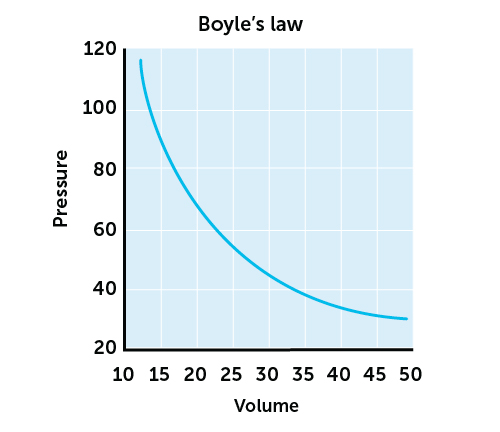
The relationship between pressure and volume of a gas means that if you halve the volume of the container, you double the pressure. If you double the volume of the container – the pressure will be halved as long as the temperature remains constant (this is Boyles Law).
As stated earlier the air pressure around you is at 760 mmHg. If you were in the Himalayas atmospheric pressure decreases as you climb up a mountain. The higher you are above sea level the less gravity pulls the gas molecules, and the greater the distance is between themolecules, therefore the pressure is lower.